High Resolution 4Pi Microscopy
Confocal fluorescence microscopy has developed into a standard tool in cell biology research; Light can easily penetrate inside the cell and furthermore, a fluorescent dye can be made to interact with specific cellular components, for example attach to an antibody that binds to a cellular protein. The resolution of confocal microscopy is ca 0.5 um laterally and 0.75 um axially.
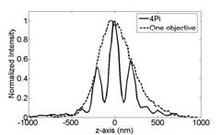
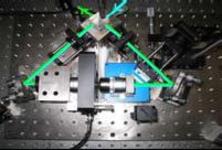
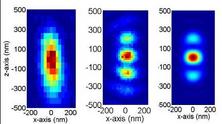
The axial resolution of a conventional confocal microscope can be improved by a factor of 3-5 in 4Pi microscopy. A 4Pi confocal fluorescence microscope uses two opposing, high numerical aperture objectives, shown in Fig 1. The counter propagating wave fronts of the illumination form interference fringes at the common focal point of the two objectives. Likewise, the collected light is interfered at the detector. This effectively reduces the axial focal volume compared to conventional confocal microscope, illustrated in Fig. 2. The measured point spread function for a fluorescent 100 nm bead is shown in figure 3. The improvement in axial resolution using two objectives (b) compared to standard confocal microscopy (1) can clearly be seen.
We are now combining 4Pi microscopy with an interferometric method we have developed, spectral self-interference fluorescent microscopy. The technique transforms the variation in emission intensity for different path lengths used in fluorescence interferometry to a variation in the intensity for different wavelengths in emission, encoding the high-resolution information in the emission spectrum. Using monolayers of streptavidin, we have demonstrated better than 5nm axial height determination for thin layers of fluorophores and built successful models that accurately fit the data.
 Physics (Internal)
Physics (Internal)