

Next: Problem 11
Up: Chapter 30
Previous: Question 14
The electron in a hydrogen atom is in the first excited state,
when the electron acquires an additional 2.86 eV of energy.
What is the quantum number n of the state into which the
electron moves?
SOLUTION:
The energy levels of the hydrogen atom are given by (eqn. 30.13)
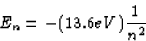
In the first excited state, n = 2, so E2 = -3.40 eV. It
acquires 2.86 eV, so
En = -3.40 eV + 2.86 eV = -0.54 eV
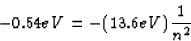
n = 5
Scott Lanning
4/15/1998
![]()
![]()