

Up: Chapter 30
Previous: Problem 8
In the line spectrum of atomic hydrogen there is also a group of
lines known as the Pfund series. These lines are produced when
electrons, excited to high energy levels, make transitions to
the n = 5 level. Determine (a) the longest wavelength and
(b) the shortest wavelength in this series. (c) Refer to Figure
24.9 and identify the region of the electromagnetic spectrum
in which these lines are found.
SOLUTION:
(a) The longest wavelength corresponds to the smallest energy, which
would occur between the n = 6 and n = 5 states. Using equation 30.14,
we get
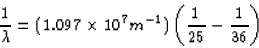
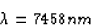
(b) The shortest wavelength is the largest energy, or when the
electron comes from n = 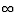 to n = 5
to n = 5
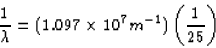
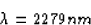
(c) The lines in the Pfund series are in the infrared.
Scott Lanning
4/15/1998
![]()
![]()
![]()
![]()