

Next: Problem 8
Up: Chapter 30
Previous: Chapter 30
Suppose that a laser produces green light instead of the red
light produced by a helium/neon laser. Are the photons emitted
by this laser more energetic, less energetic, or of equal energy
as compared to those emitted by the helium/neon laser? Justify
your answer.
SOLUTION:
Green light has a shorter wavelength than red light (c.f.,
Table 26.2). So what? Well, we can express the energy of a photon
in terms of its wavelength. First, we know by the Planck quantization
relation that
E = hf .
We also know that
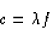
if you recall equation 16.1. (Oh, you forgot?!? Well, speed is distance
per time, and wavelength is a distance and frequency is inverse
period or inverse time.) Combining, we get
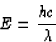
so we see that, since green light has a shorter wavelength than
red light, its energy is larger.
Scott Lanning
4/15/1998
![]()
![]()