

Next: Chapter 32
Up: Chapter 31
Previous: Problem 37
Find the energy (in MeV) released when 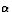 decay converts
radium 22688Ra (atomic mass = 226.02540 u) into radon
22686Rn (atomic mass = 222.01757 u).
decay converts
radium 22688Ra (atomic mass = 226.02540 u) into radon
22686Rn (atomic mass = 222.01757 u).
SOLUTION: Use Energy Conservation. By ``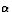 decay converts'',
we mean that the parent particle turns into an alpha particle and
daughter particles. Adding the mass of the alpha and daughter radon,
we get
decay converts'',
we mean that the parent particle turns into an alpha particle and
daughter particles. Adding the mass of the alpha and daughter radon,
we get
m = 4.00260 u + 222.01757 u = 226.02017 u .
The parent had a mass of 226.02540 u, so clearly some mass has
gone somewhere. The amount of the missing mass is
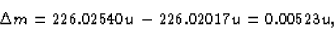
which is equivalent to an energy change of
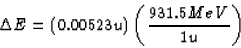
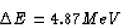
Scott Lanning
4/23/1998
![]()
![]()
![]()