

Next: Problem 37
Up: Chapter 31
Previous: Question 13
Iodine 13153I is used in diagnostic and therapeutic techniques
in the treatment of thyroid disorders. This isotope has a half-life
of 8.04 days. What percentage of an initial sample of 13153I
remains after 30.0 days?
SOLUTION: Radioactive decay is exponential; that is, if No is the
original number of particles, and N(t) is the number of particles at
time t, then
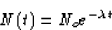
Half-life T1/2 is defined as the time it takes for there to be
remaining half the original number of particles; that is,
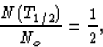
and, combining with the first equation,
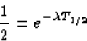
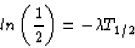

(since ln(a/b) = ln(a) - ln(b), and ln(1) = 0). So, we see that
we can relate the decay constant to the half-life
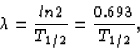
and, in our case,
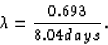
We want the percentage of the original sample remaining after
30 days, or, in other words, we want the ratio N(30 days)/No.
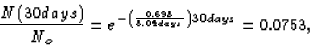
so the percentage remaining is 7.53%.
Scott Lanning
4/23/1998
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()