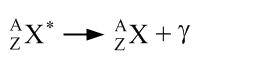
The third class of radioactive decay is gamma decay, in which the nucleus changes from a higher-level energy state to a lower level. Similar to the energy levels for electrons in the atom, the nucleus has energy levels. The concepts of shells, and more stable nuclei having filled shells, apply to the nucleus as well.
When an electron changes levels, the energy involved is usually a few eV, so a visible or ultraviolet photon is emitted. In the nucleus, energy differences between levels are much larger, typically a few hundred keV, so the photon emitted is a gamma ray. Gamma rays are very penetrating; they can be most efficiently absorbed by a relatively thick layer of high-density material such as lead.
A gamma decay is written in the following way:
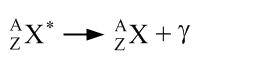
The asterisk indicates that the nucleus is in an excited state. Note that this is the only decay in which the atom does not become another element.