Q9) The form of Coulomb's law is very similar to that for Newton's
law of universal gravitation.
What are the differences between
these two laws? Compare also gravitational mass and electric charge.
SOLUTION:The gravitational force is always attractive, and the magnitude of the force is: Gm1m2/r2. The electric force is attractive if the charges have opposite signs, and is repulsive if the charges have the same sign. The force magnitude is: kq1q2/r2. The gravitational and electric force are very similar in each having the inverse distance- squared rule, and in having the magnitudes depending in a linear and symmetric fashion on properties of the two objects. The major difference is that possibility of repulsion for the electric force.
P2)Two charged smoke particles exert a force of 4.2 x 10-2 N on each other. What will be the force if they are moved so they are only one eighth as far apart?
SOLUTION:
F=kq1q2/r2 =
4.2 x10-2 N If r ->
(1/8)r, r2 -> (1/64)r2 and so,
F = 64(4.2 x 10-2 N) = 1.69 N
P6)What is the repulsive electrical force between two protons in a nucleus that are 5.0 x10-15 m apart?
SOLUTION:
F = (9 x
109 Nm2/C2)(1.6 x 10-19
C)2 / (5.0 x10-15 m)2 = 9.2 N
P8)A person scuffing her feet on a wool rug on a dry day accumulates a
net charge of -60 µC.
How many excess electrons does this person
get, and by how much does her mass increase?
SOLUTION:
Q = -60 µC, so the number of electrons is the total
charge divided by the charge per electron:
Ne = Q / (-e) = 60
µC/1.6x10-19 C
=
60 x10-6/1.6x10-19 = 3.75 x
1014 excess electrons.
The additional mass due to the excess electrons is the number of
electrons multiplied by
the mass per electron:
Ne( 9.11 x
10-31kg) = 3.42 x10-16 kg
P11)Particles of charge +70, +48, and -80 µC are placed in a line.
The center one is 0.35m from
each of the others. Calculate the net
force on each due to the other two (see Fig. 16-37 in text book).
SOLUTION:
Let Q1 = 70 µC ,
Q2 = 48 µC,
Q3 = -80 µC
Let the x axis lie
along the charges. We will calculate the x component of the forces (the
other components vanish).
F1x = F12x + F13x = -k
| Q1Q2 | / (0.35 m)2 + k
| Q1Q3 | / (0.70
m)2
F1x = [(9.0 x
109 Nm2/C2) / (0.70 m)2]
x (70
µC) [-4 (48µC) + 80 µC]
= -144
N (force is to the left, indicated by negative value)
F2x = F21x + F23x = k
| Q1Q2 | / (0.35 m)2 + k
| Q2Q3 | / (0.35 m)2
F2x = [(9.0 x
109 Nm2/C2) / (0.35 m)2]
x (48
µC) [ (70µC) + 80 µC]
= 529
N (force is to the right, indicated by positive value)
F3x = F31x + F32x = -k
| Q1Q3 | / (0.70 m)2 - k
| Q2Q3 | / (0.35 m)2
F3x = [(9.0 x
109 Nm2/C2) / (0.70 m)2]
x (80
µC) [ (-70µC) - 48 µC x 4]
= 385
N (force is to the left, indicated by positive value)
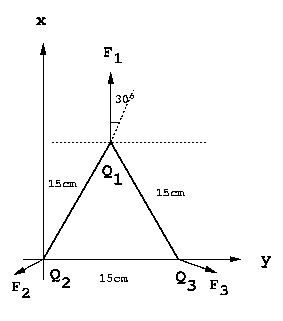
P12)Three positive particles of charges 11.0 µC are located at the corners of an equilateral triangle of side 15.0 cm (see Fig. 16-38 in text book). Calculate the magnitude and direction of the net force on each particle.
SOLUTION:

Due to symmetry, each force is directed
perpendicular to and away from the lines joining the other two charges.
To calculate the magnitude, we only need consider one case. For
simplicity, the best case is, F1 , since it is apparent that
F1x = 0.
F1y = F12y + F13y = | F12 |
cos30 + | F13 | cos30 = 2 x |F12 |
cos30
since the magnitude of the vector | F12 | = | F13 |
| F1y | = 2 x [k (11
µC)2 / (0.15 m)2] x (31/2/2)
= 83.8 N so, | F1 | = |
F2 | = | F3 | = 83.8 N
P15)Compare the electric force holding the electron in orbit around the
proton (r = 0.53 x 10-10m) in the hydrogen nucleus with
the gravitational force between the same electron and proton. What is
the ratio
of these two forces?
SOLUTION:
Electric force magnitude:
FE = (9 x
109 Nm2/C2)(1.6 x 10-19
C)2 / (0.53 x
10-10m)2
= 8.20 x 10-8 N
Gravitational force magnitude:
FG = (6.67 x
10-11 Nm2/kg2)(9.0 10-31
kg) (1.67 x 10-27kg) / (0.53 x 10-10m)2
= 3.61 x 10-47 N
The electric force is much stronger, in the ratio
FE / FG = 2.3 x 1039
P18)In one model of the hydrogen atom, the electron revolves in a circular orbit around the proton with a speed of 1.1 x 106 m/s. Determine the radius of the electron's orbit.
SOLUTION:
The centripetal force which keeps the
electron in orbit is provided by the electric force.
mv2/r = ke2 / r2
so, r = ke2 /
mv2 = [(9 x
109 Nm2/C2)(1.6 x 10-19
C)2] / [(9.11 x 10-31
kg) x
(1.1 x 106
m/s)2] = 2.09 x 10-10m